R&D KOSÉ Corporation Identifies a Gene, EFEMP2, Which Regulates the Senescence Fate of Dermal Fibroblasts 2023.11.21 Show Full [ PDF / 867KB ]
KOSÉ Corporation (Headquarters: Chuo-ku, Tokyo; President: Kazutoshi Kobayashi), in collaborative research with Associate Professor Daisuke Namba of the Division of Aging and Regeneration, The Institute of Medical Science, The University of Tokyo (affiliated with Tokyo Medical and Dental University at the time of the research), have established that the EFEMP2 gene functions as one of the genes which regulate the “cell fate” (presumptive differentiation pathway) determining whether dermal fibroblasts will become senescent. Since dermal fibroblasts produce collagen, elastin and other proteins comprised in the skin and are also involved in maintaining skin firmness and elasticity, this finding is a major breakthrough forward in skin rejuvenation research. Part of the results of this research were published online in September 2023 in the scientific journal Experimental Dermatology, the official dermatology journal of the Dermatological Research Working Group (ADF).
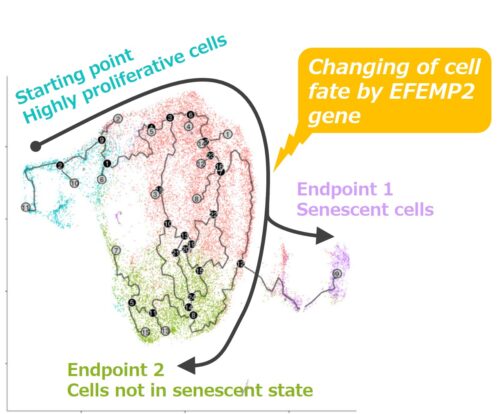
Fig. 1: Analysis results of population distribution and presumptive differentiation pathway (cell fate) of dermal fibroblasts
Background
Aging-related changes in skin such as wrinkles and sagging are concerns for many people. By conducting research to elucidate the mechanisms of this aging, we aim to provide effective solutions to our customers for dealing with these concerns.
In recent years, researchers have focused on the finding that, as senescent cells accumulate in the body with aging, they also age the surrounding cells and tissues. Fibroblasts, which are present in the dermis and produce collagen and elastin, also cause the surrounding cells to age and reduce their functionality, which is an important research issue. In order to prevent this chain reaction of senescent cells, it is considered effective to analyze the processes that transform cells into senescent cells and find methods of decreasing the occurrence of senescent cells.
On the other hand, it is not easy to evaluate cell changes due to aging. When comparing cells of people of different ages, there are not only genetic differences but also large individual differences due to lifestyle habits such as the degree of ultraviolet radiation exposure, eating and drinking habits, and it is difficult to separate them from the effects of aging. Therefore, we used a sequence of dermal fibroblasts collected from the same person over a period of 35 years to enable minimization of such impacts in order to search for new findings on the excessive accumulation of senescent cells due to aging.
Inference of changing cell fate of fibroblasts due to aging
Fibroblasts consist of multiple cell populations with different properties and it is thought that the state of each cell population changes with aging. Focusing our attention on this, and using a technique called single-cell RNA sequencing, which can analyze the gene expression of each cell, we analyzed how changes in aging in the same person over more than 35 years can cause changes in the cell population.
Based on the patterns of gene expression, fibroblasts were broadly divided into four groups: (1) highly proliferative cells, (2) cells with high production of extracellular matrix*1, (3) senescent cells, and (4) cells that were not in a senescent state. The main changes that were indicated in fibroblasts due to aging were an increase in senescent cells, a decrease in highly proliferative cells, and a decrease in functions such as the production of extracellular matrix. For example, when comparing fibroblasts at ages 36 and 72, results were that the proportion of senescent cells increased from approximately 4% to approximately 10%, and the proportion of highly proliferative cells decreased from approximately 15% to approximately 4% (Fig. 2).
*1 Extracellular matrix: Structure made of collagen and other proteins that fills the area surrounding cells
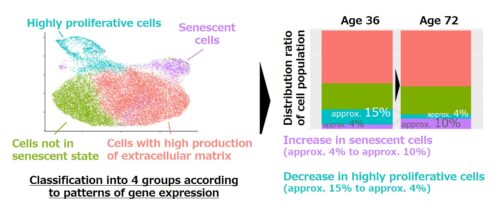
Fig. 2: Analysis results of single-cell RNA sequencing analysis of the same person’s dermal fibroblasts (derived from cells at ages 36 and 72)
Identification of EFEMP2 gene that changes the senescence fate of dermal fibroblasts
Using a technique called pseudotime analysis, and based on gene expression patterns, we analyzed the sorts of changes in cell conditions that follow on with aging in a cell population with high proliferative activity that was set as the starting point. As a result, the existence of two presumptive differentiation pathways (cell fates) was inferred, with senescent cells as an endpoint (endpoint 1) and cells not in the senescent state as the other endpoint (endpoint 2) (Fig. 1). Thereafter, focusing on the EFEMP2 gene characteristic of endpoint 2 cells not in the senescent state, the cell senescence state when the expression of the gene was not suppressed was evaluated. As a result, fibroblasts in which the expression of EFEMP2 was suppressed showed an increase in cells exhibiting characteristics of senescent cells, indicating that EFEMP2 is involved in changing the senescence fate of fibroblasts (Fig. 3).This increase in senescent cells due to the suppression of EFEMP2 has also been found in dermal fibroblasts derived from other cell donors.
The EFEMP2 gene produces a protein called fibulin-4 which is important for collagen and elastin formation but this research is the first report of its direct relationship with senescent cells.
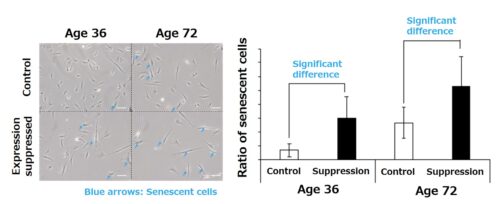
Fig. 3: Analysis of the senescence state of dermal fibroblasts when the EFEMP2 gene is suppressed
Future outlook
Through this research, KOSÉ Corporation was able to obtain valuable knowledge about senescent cells that accumulate with aging which will lead to the development of new solutions. The company will continue to engage in rejuvenation research, such as the elucidation of aging mechanisms, with the aim of contributing to healthy and comfortable lifestyles through the power of beauty.
Share